Paxlovid, "Snake Oil" of the 21st Century?
Paxlovid Clears Symptoms, but does it Clear the Virus?
From this site:
Paxlovid is a combination of a protease inhibitor Nirmatrelvir and a HIV medication Ritonavir. At $895, it is definitely going to be a moneymaker for Pfizer.
But how well does it work for the patients?
This is what we all heard:
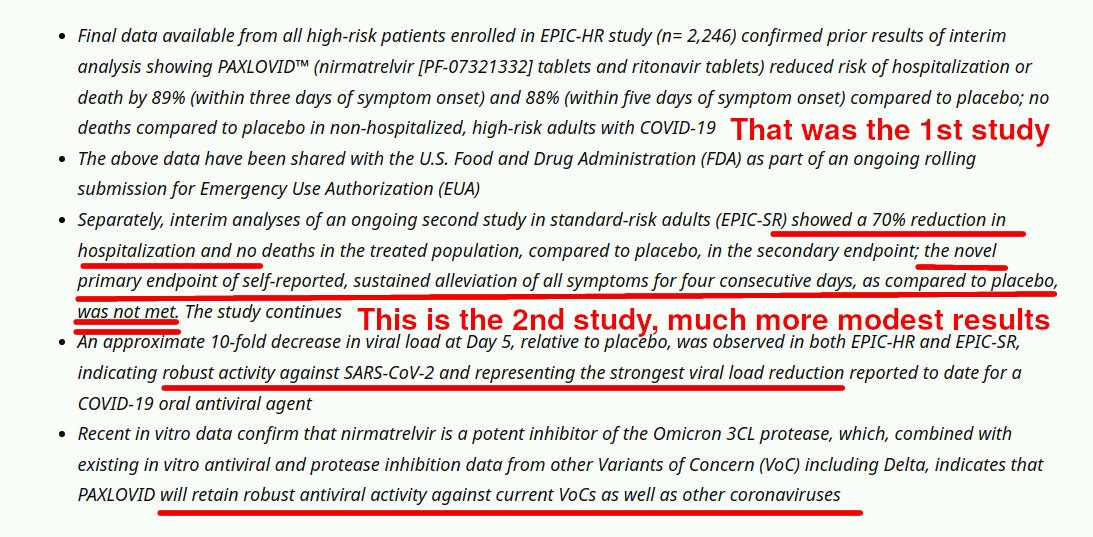
The first study, that lasted for four weeks only, reported amazing success and “89% prevention of severe symptoms”. That first study was enough to get FDA to give Paxlovid a EUA.
Who cares that the subsequent results, that actually appeared before the FDA decision but after Pfizer submission, showed a much worse performance:
This is pretty typical for another famous Pfizer product, right? We all heard how it was “99% effective”, then “95%”, then “43%” etc — you know how it worked out.
Same with Paxlovid: first it was 89%, then 70%, failed to achieve sustained alleviation of symptoms, etc.
How does it work for actual people?
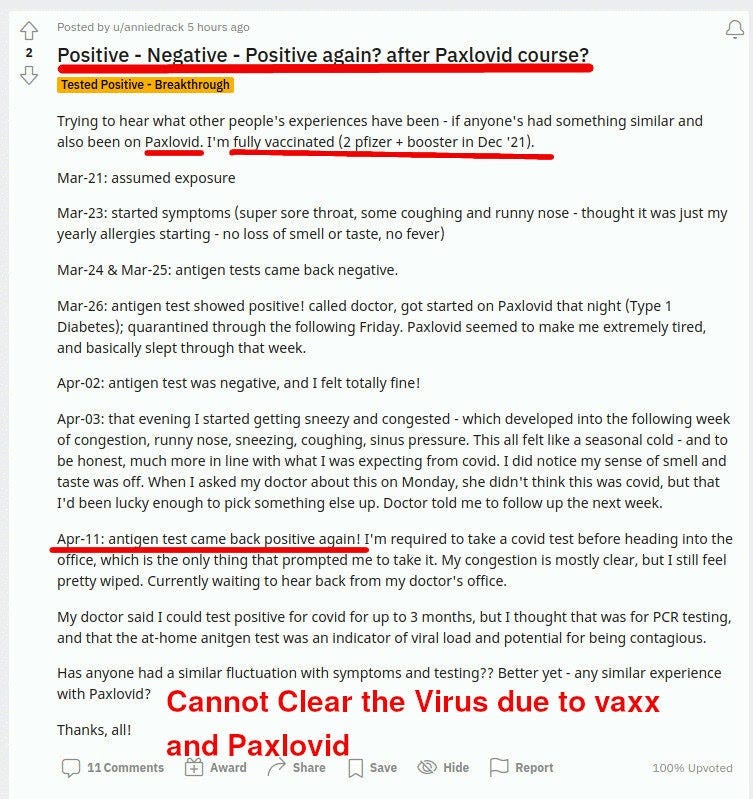
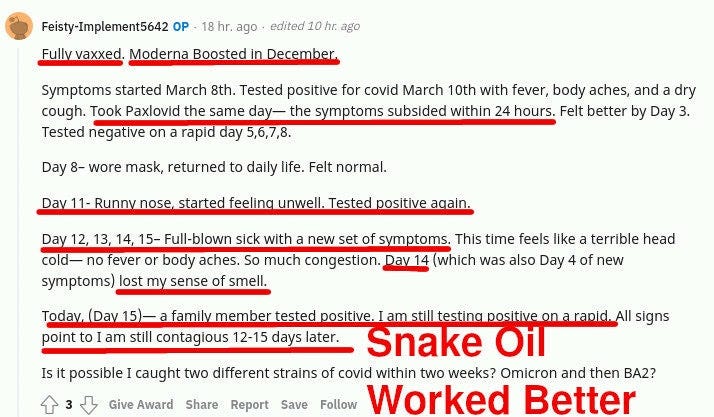
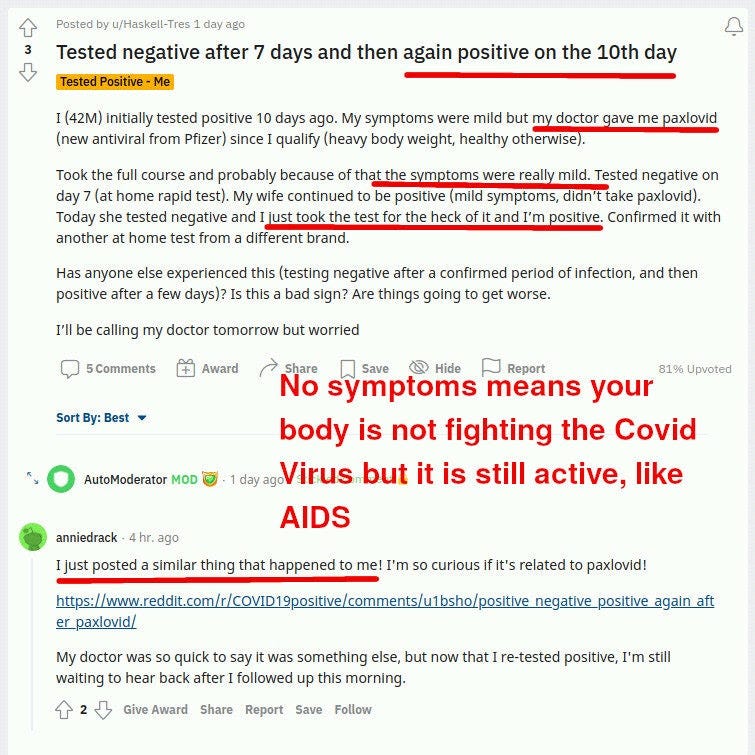
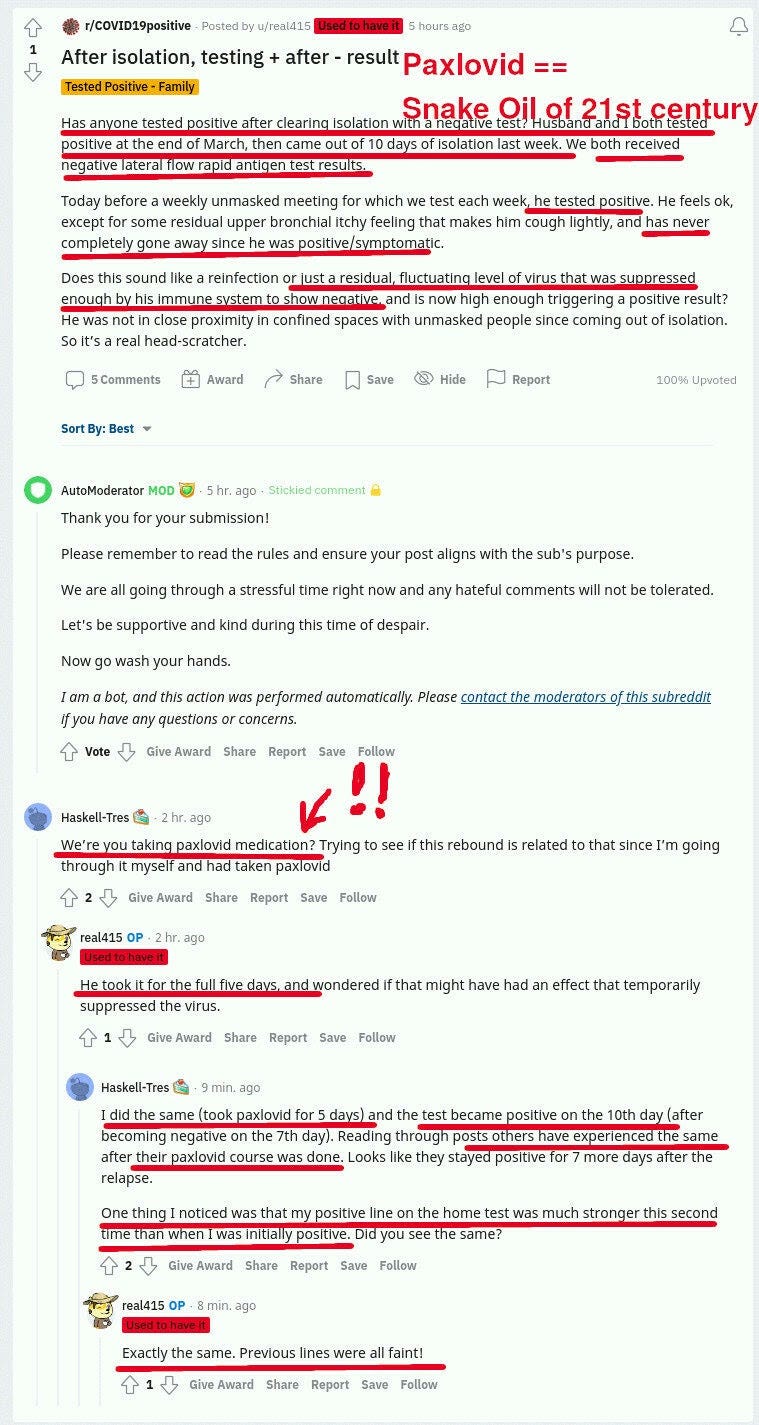
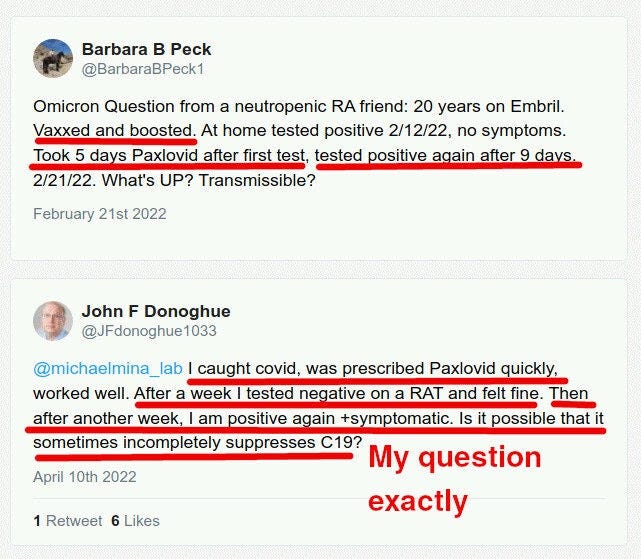
It turns out that Paxlovid reduces symptoms, and maybe reduces viral load at day 5, but at the same time it does not clear the virus! Not only that, but the patients cannot clear it for extended periods AFTER paxlovid administration is over:
In the reddit posts above, you can see a few redditors stumped about this and sharing what is happening to them, trying to make sense of what is happening.
Here are some Twitter posts:
These people report stronger RAT test results — thicker lines — after taking Paxlovid. One reported infecting a family member around Day 15.
Just to remind everyone, we have “symptoms”, such as fever, because our immune system is working to counter the infection and kill off the pathogen.
Having no symptoms and no infection is a good thing. Having no symptoms and an ongoing, contagious Covid infection is a bad thing.
Does Paxlovid turn people into asymptomatic Covid superspreaders? It is a question that we cannot answer, and that need to be assessed and addressed by science. Will anyone care? I doubt, but I hope that someone important takes notice.